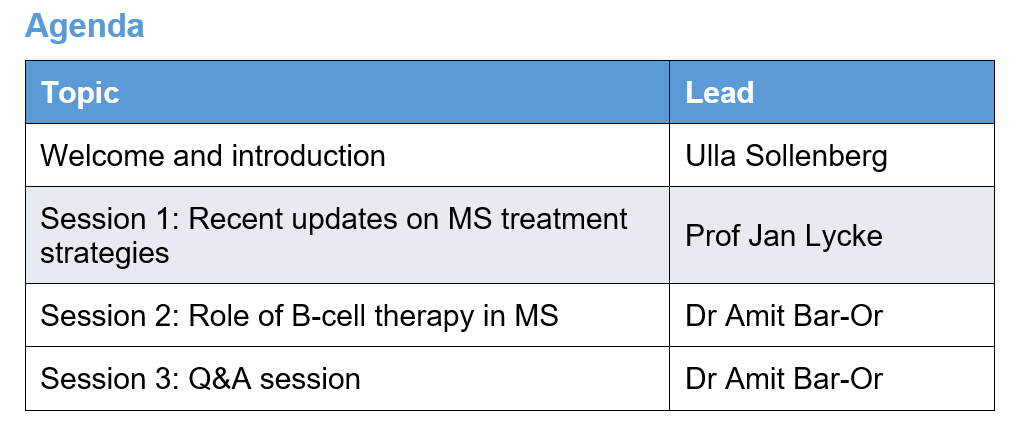
Session 1: Recent updates on MS treatment strategies
Speaker: Prof Jan Lycke
Key highlights
Disease-modifying therapies in MS act by modulating or suppressing the immune system, though some DMTs may also influence neurodegeneration. In the early stages of MS, pathology is dominated by inflammation, but as MS progresses neurodegeneration becomes more prominent due to oxidative injury that impairs mitochondrial function and results in axonal injury. Hence, MS treatment strategies may focus on components like inflammation and neurodegeneration.
Key clinical challenges in MS treatment:
- Limited understanding of the MS pathogenesis
- Is neurodegeneration primary or secondary to inflammation?
- Is systemic immunomodulation or immunosuppression sufficient?
- Is it important to reduce compartmentalised inflammation within the CNS?
- Is it possible to influence remyelination and regeneration?
- Should treatment also affect innate immunity?
- Is using combination therapies the next step?
Current treatment strategies include escalation therapy, high-efficacy DMT from the onset and immune reconstitution therapy.The cohort study data on 4861 patients with MS from Denmark (n=2161) versus Sweden (n=2700) suggest that early treatment using more efficacious DMT was superior to escalation therapy. The Swedish treatment strategy was associated with significant reduction (29%) in the rate of 24-week confirmed disability worsening and relapse outcomes (56%) relative to the Danish treatment strategy.1
Session 2: Role of B cell therapy in MS
Speaker: Dr Amit Bar-Or
Key highlights
Traditionally, in MS, there is abnormal antibody production in the CNS. B cells are important for the initiation and perpetuation of CNS-directed inflammatory activity. B cells play a diverse role in MS. It was proposed that antigen presentation and cytokine production are the relevant roles of B cells that help us understand MS relapse biology. Abnormal B-cell cytokine responses mediate ‘bystander activation’ of disease-relevant proinflammatory T cells, resulting in new relapsing MS disease activity. Thus, B cells are major checkpoints contributing to regulating inflammatory signals.2,3
MS and anti-CD20 therapies during the COVID-19 pandemic
As per the large-scale analysis, MS diagnosis was unlikely to increase the risk of severe COVID-19 outcomes, although few researchers have predicted worse outcomes, particularly for those taking immunosuppressive DMTs. The reported mechanisms of COVID-19 severity in MS patients are listed below:
- Severe COVID-19 outcomes in patients with MS appear to be influenced by age, comorbidities (obesity, diabetes and pre-existing cardiovascular or lung disease) and a high level of disability (EDSS score >6) or progressive forms of MS.4,5,6
- A pooled COVISEP study group analysis from Italy and France confirms an increased risk of COVID-19 severity outcomes in patients on anti-CD20 and methylprednisolone therapies (P < .001), whereas interferons may be associated with a decreased risk.7
COVID-19 vaccine response in the context of DMTs is affected by several factors, such as the SARS-CoV-2 virus variant, type of vaccine (mRNA or non-replicating viral vector) and MOA of DMT.
Practice and evidence-based recommendations
- DMTs may have different effects on vaccine response depending on the MOA and the time of administration.
- Vaccine-induced neutralising antibodies to spike protein are probably important for effective protection from SARS-CoV-2 infection.
- B-cell therapies may not impact pre-existing humoral immunity but are expected to attenuate vaccine-induced antibody responses.
- The VELOCE study data provide class II evidence confirming that the humoral response to non-live vaccines is reduced in patients with relapsing MS after ocrelizumab treatment compared with untreated or interferon beta–treated patients.8
- Post-vaccination serological assessments reported by Sormani et al9 are summarised below:
- the antibody titres after vaccination with mRNA-1273 (Moderna) are higher than those after vaccination with BNT162b2 (Pfizer/BioNTech).
- the antibody levels of patients on ocrelizumab (201-fold decrease) and fingolimod (26-fold decrease) were reduced as compared with those of untreated patients.
- time to anti-CD20 infusion matters; time span between the last infusion and the vaccination may be necessary to have an antibody response to vaccine.
- the above findings may have an impact on the choice of the vaccine for patients treated with anti-CD20 and fingolimod.
- MS patients treated with anti-CD20 generated antigen-specific CD4 and CD8 T-cell responses after vaccination. The findings may have implications for clinical decision-making and public health policy for immunocompromised patients, including those treated with anti-CD20, and sheds light on in vivo B-cell, CD4 and CD8 T-cell interactions.
Abbreviations
CNS, Central nervous system
COVISEP, Epidemiological characteristics of coronavirus infection (SARS-CoV-2) in patients with MS
CD, Cluster of differentiation
DMT, Disease-modifying therapy
EDSS, Expanded Disability Status Scale
mRNA, Messenger ribonucleic acid
MS, Multiple sclerosis
MOA, Mechanism of action
US, United States
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
VELOCE, A Study to Evaluate the Effects of Ocrelizumab on Immune Responses in Participants with Relapsing Forms of Multiple Sclerosis
References
-
Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197–1204.
-
Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696–707.
-
Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 2021;20(6):470–483.
-
Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2021;49:102725.
-
Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics, and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088.
-
Multiple Sclerosis & COVID-19.https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus Accessed on February 10, 2022.
-
Sormani MP, Salvetti M, Labauge P, et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. 2021;8(8):1738–1744.
-
Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–e2008.
-
Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBio Medicine. 2021;72:103581.
-
Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001.