Migraine constitutes a significant burden, not only to the individual suffering from this disabling disease but also to the society via sick leaves and use of health care resources. Two recent registry studies outline the burden of migraine in a Nordic setting and highlight the potential of erenumab, the first monoclonal antibody1 developed for prevention of migraine, to lower the burden of headache-related societal costs.
Since 1990, migraine has been one of the top five leading causes for disability worldwide, and in 2016 it was ranked the number one cause in people below 502. An extensive Finnish study1 recently put these global numbers into a Nordic perspective. Here, data from 17,623 individuals with migraine from a registry of occupational health care users* not only confirmed that migraine limits the lives of people living with the disease but also demonstrated its broader impact at a societal level3.
High unmet need among patients with treatment unresponsive migraine
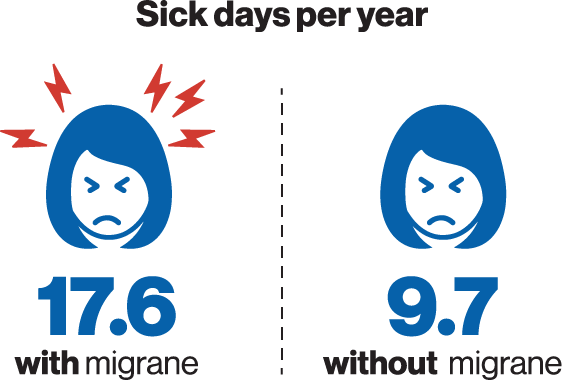
The highest proportion of people living with migraine were found among women of prime working age. In line with the disabling nature of the disease, people with migraine had almost twice as many sick days (17.6 per year) as compared to matching controls without migraine (9.7 per year). People living with migraine use of healthcare resources was also markedly higher (14.4 visits per year) as compared to that of people without migraine (8.5 visits per year)4.
A closer look at the data reveals some discrepancies between the individual subgroups of migraine patients. Whereas most of the migraine patients who were not on medication or took only acute medication had an average of 16-17 sick leave days a year, those on preventive migraine treatment (suggestive of chronic and more severe migraine) were on sick leave more than 22 days a year on average. Of note, preventive medications prescribed were not developed for migraine and included beta-blockers, antidepressants, and others4.
The recurrent sick days and health care visits mirror a high unmet need among people with migraine. Especially those suffering from unresponsive treatment or refractory migraine have a need for improved migraine management.
Erenumab – a monoclonal antibody treatment developed for migraine prevention
Recently, a option for patients with migraine has been provided. Clinical trials have shown that erenumab, a monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) pathway, reduces the number of monthly migraine days in patients with episodic or chronic migraine1,4.
To elucidate whether the clinical trial results could translate into fewer sick leave days and health care visits in a real-world setting, data from 82 patients with migraine who were treated with erenumab were extracted from the Finnish registry of occupational health care users*4. Included patients had at least eight migraine days a month and had failed on two or more preventive treatments. Also, because of the Finnish reimbursement criteria, only patients who responded to erenumab with a ≥50% reduction in monthly migraine days were studied. As compared to one year before initiation of erenumab, the number of migraine-related sick leave days were lowered by 74% one year after erenumab onset4. In exact numbers, the patients went from an average of 4.9 sick leave days per year before erenumab to 1.3 sick leave days annually one year after erenumab4. See Figure. Where about one in three had at least one sick leave day a year due to headache, this proportion decreased to about one in six following the initiation of erenumab4.
The number of health care visits related to headache diminished from 4.9 visits before to 2.7 visits after, corresponding to a reduction of 45%4. This was also reflected in the fraction of patients with five or more annual headache-related visits which decreased from about 40% to about 16% after initiation of erenumab treatment4.
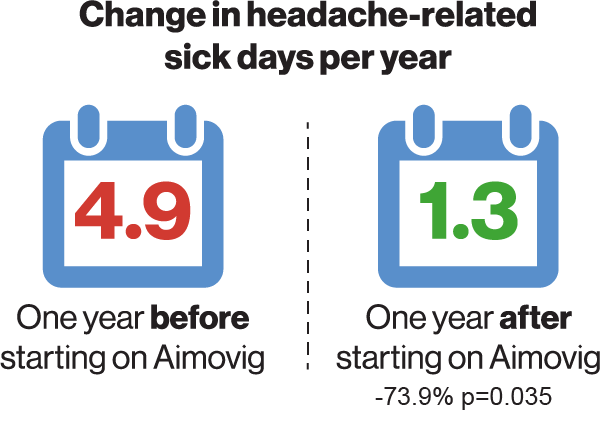
Change in headache-related sick leave days in patients on erenumab treatment and in age- and gender-matched controls. P value compared to reference (12 months before initiation of treatment)4.
Erenumab treatment also led to a diminished need for acute migraine medication. The use of triptans was reduced by 30% after the initiation of erenumab4, and similar reductions were observed in the erenumab registrational studies1.
In contrast, no changes were observed for other pain medications. In conclusion, the study implies that erenumab may aid in reducing sick days and the need for acute treatment.
Read the full-length articles here:
https://doi.org/10.1186/s10194-019-0964-5
https://doi.org/10.1007/s40120-021-00303-x
Erenumab Safety profile: Common side effects: hypersensitivity reactions, injection site reactions, constipation, muscle spasms, and pruritus1.
1. Aimovig SmPC, chapter 4.8, 5.1
2. Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain 2018;19(1):17 https://doi.org/10.1186/s10194-018-0846-2.
3. Korolainen MA et al. Burden of migraine in Finland: health care resource use, sick-leaves and comorbidities in occupational health care. J Headache Pain. 2019 Feb 12;20(1):13.
4. Autio H et al. Erenumab Decreases HeadacheRelated Sick Leave Days and Health Care Visits: A Retrospective Real-World Study in Working Patients with Migraine. Neurol Ther. 2021 Dec 10:1–13. https://doi.org/10.1007/s40120-021-00303-x
*Electronic medical records from the private health care provider Terveystalo were utilized
